We’ve covered the components of an Arterial Blood Gas test, discussed pH, and the partial pressure of gases. I promise, this will all make sense at the end. I know there are mnemonics and tables and tricks to tell you what type of acid-base disorder your patient has. These are fine. But truly understanding the science behind these disorders will make your clinical practice better. Stick with it; you’re doing great!
Back to Basics
Alright. We have a grasp on pH and acid versus basic. We know that oxygen (O2) and carbon dioxide (CO2) are carried on red blood cells via hemoglobin as well as dissolved like soda bubbles in the blood. We know that increasing the ratio of O2 a person inhales will impact how much O2 is pushed into their blood.
Let’s do a quick review of the chemicals involved and their normal ranges. Your facility may have slightly different values based on your equipment, but they should be similar.
pH = 7.35 - 7.45
A body at equilibrium will maintain this through absorption and excretion of bicarbonate (HCO3) and hydrogen ions (H+). It does this through the kidneys (absorption and excretion) and the lungs (by expiring CO2).
O2 does not affect pH.
PaO2 = 75 - 100 mmHg
I know it’s annoying that the PaO2 and the SpO2 are not the same range. That’s because PaO2 is measuring a pressure (mmHG) and SpO2 measures percentage (%).
This is a measure of how much O2 is getting from the lungs into the blood. Lots of things can affect this process, which we will discuss later.
This is the number I use to judge if the patient might need intubation.
More is not better in this case. Carrying greater than 100 mmHg PaO2 is a sign of over over-ventilation or over-oxygenation. Remember, O2 is a chemical just like any other medication. Only use the effective dose!
PaCO2 = 35 - 45 mmHG
This is a measure of how much CO2 the body is getting from the blood into the lungs.
This number can vary widely due to chronic and acute processes. And same as O2, many things can change this number.
This is the number I use to judge if the patient needs more pressure.
HCO3 = 22 - 26 mEq/L
HCO3 will absorb H+, converting to H2CO3, as part of the body’s buffering system.
Yes, that’s the same stuff you use to make baking soda volcanoes.
I use this number to judge if the acid-base disorder is metabolic in origin, meaning something not lung-related.
Base Excess = -2 - +2 mmol/L
This has to do with determining metabolic disorders as well. We’ll cover it in a later Nurse Files.
Basically tells you how out of whack everything else is.
Bedside Considerations
In the last Nurse Files, I presented a case study of an elderly person with acute hypoxia. They had increasing oxygen demand, requiring 6 liters per minute (lpm) of oxygen supplementation.
We know that each liter of oxygen increases the ratio of oxygen by 4%. Going from room air — 21% — to 6 lpm — 45% — is a significant change in status. Our patient is exhibiting signs of hypoxia with an increased heartrate and respiratory rate, as well as the increased O2 demand. Their symptoms include shortness of breath and increased work of breathing.
In the setting of a known respiratory infection, with no other complications such as a high fever or anxiety attack, I will assume this patient’s distress is respiratory related. Thus, I need to intervene in a way to support their respiratory system before it loses the ability to compensate.
I would ask for an ABG to evaluate what intervention this person needs: pressure or flow. I can do this by evaluating the numbers the ABG gives me and seeing what is out of whack. We’ll focus on the respiratory numbers for now.
Let’s say our numbers come back pH = 7.33, PaO2 = 60, PaCO2 = 37
Okay, one at a time:
The pH is below normal, but not wildly out of range. I am not concerned for acute complications based solely on the pH. Thus, I don’t think their increased respiratory rate is an attempt by their lungs to compensate for a metabolic acid-base disorder.
PaO2 is low. Arterial blood is either not picking up enough O2 from the lungs or the O2 demand at the cellular level is increased, using more than the lungs can replenish.
PaCO2 is normal, if on the low end and might be due to the increased respiratory rate. Breathing faster will pull in more O2, yes, but also let more CO2 out.
Now, with the low PaO2, the first step would be to increase the amount of O2 they are inhaling. In fact, this is what this percentage is called, the fraction of inspired oxygen or FiO2. Another way to say ‘room air’ is to say FiO2 of 21%.
But we’ve already tried that. We’ve gone from room air or FiO2 of 21% to an FiO2 of nearly 50% and the PaO2 is still low. That’s bad. We’ll need to find out why this is happening, but first we need to stabilize the patient.
We gave a nebulizer, sat them up nice and tall, treated their fever, and they are still having issues. We’ve gotten our ABG, showing that something isn’t right. What do we do next?
I want a chest x-ray. I want to see if something has acutely changed. Are they in fluid overload? Have they been coughing and developed a pneumothorax? Do they have a pulmonary embolism? We need to identify where the problem is and the mechanics of it.
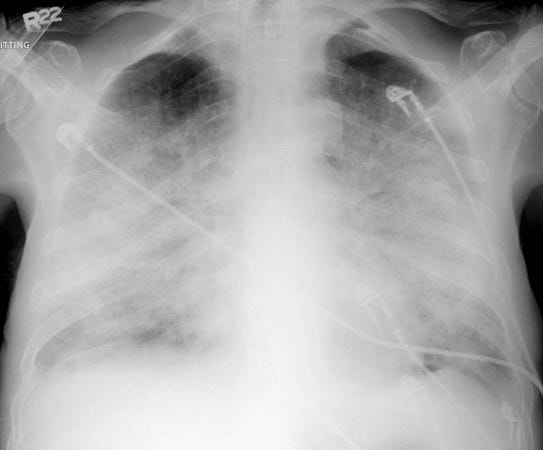
We get our x-ray and it looks like this:
I hope you all just cringed. This x-ray looks terrible. No wonder they can’t breathe. They’ve developed massive pulmonary edema. If I was strolling by and saw this pop up on the portable x-ray machine, I’d guess they were dehydrated when they came in due to their pneumonia. Maybe had an increased lactic level and thus were rehydrated. Their older lungs and heart couldn’t handle the fluid and it had to go somewhere.
This person needs diuresis, but in the meantime, I want to increase the amount of oxygen they are absorbing through the lung tissue that is working. Increasing the flow and/or pressure would do this. If they can tolerate, I’d place a BiPap. Even an hour or two would help them get through this acute respiratory distress until the edema can be addressed.
This is one example. This scenario can play out with many variables, as our patients can have any combination of risk factors, health issues, and complications. As a nurse, your most pressing concern is to keep the body’s natural compensation processes from failing. We need to stop distress from progressing to failure. Early identification and mobilization of your resources is key.
Don’t be afraid to ask for help. I’d rather lend a hand giving a nebulizer than doing CPR. I don’t get nearly so sweaty.
Keep at it! This is complicated topic. You’ve done a good job so far. A few more installments and you’ll be interpreting your ABGs — and treating your patients — like a champ.
References
Porth’s Pathophysiology: Concepts of Altered Health States (9th Edition). Grossman, S.C., Porth, C.M.
Thank you for reading! You can follow me on Instagram and subscribe to this newsletter to stay up to date with posts, practice NCLEX questions, and my stories of life as an inpatient nurse.
If you found this article interesting, useful, or entertaining, please consider donating via a subscription. All This Is My Nurse Face content is free, but you can choose a subscription to help support my writing. My goal is to educate and inspire healthcare professionals and laymen alike. Your support helps me achieve that!
Also, please check out my other Substack: WritingRampant. No nursing, but occasional blood and guts. (My characters like to stab each other. Such drama queens...)
Enjoy!
Anna, RN, BSN, CCRN
Necessary disclaimer: I am discussing medications and medical conditions in this article based on my personal experiences as a nurse. Your facility may have different requirements and resources. Use your own nursing judgement to assess and treat your patients according to your governing body and facility guidelines. All information within this article is correct to the best of my knowledge, but should be confirmed through verified evidence-based sources. I am not responsible for any clinical decisions made based on this article.